Are Varicose Veins Genetic?
Aira
on
August 10, 2024
What Are Varicose Veins?
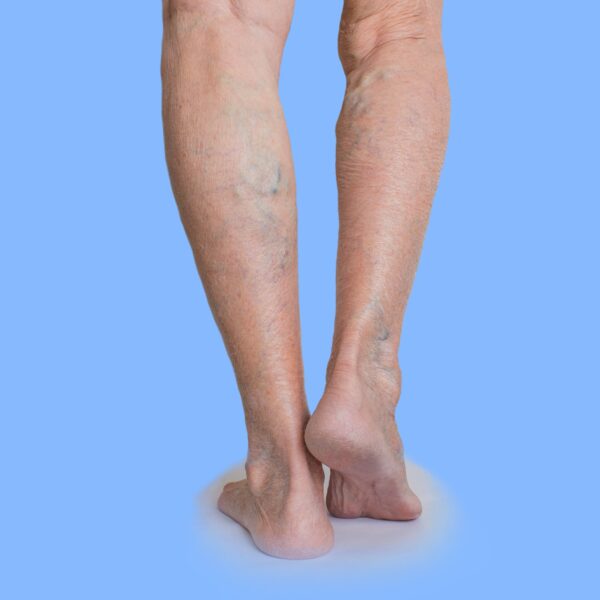
Varicose veins are a common condition of the blood vessels characterized by swollen, enlarged, and twisted veins visible beneath the skin’s surface. Most frequently occurring in the legs and feet, these veins can range from dark purple to blue. Though varicose veins can be unsightly, they often pose more than just a cosmetic problem; they can lead to discomfort, pain, and sometimes severe medical issues related to the circulatory system.
A healthy vein allows blood to flow smoothly back to the heart through a series of one-way valves. In a varicose vein, these valves malfunction, allowing blood to accumulate, leading to enlargement and a twisted, rope-like appearance. While smaller veins are often red or purple, more prominent varicose veins tend to be bluish.
Are Varicose Veins Genetic?
While varicose veins can be influenced by factors like age, weight, and lifestyle, there could also be a genetic component.
Research on Genetic Markers
Studies on the genetic aspects of varicose veins have mainly focused on symptoms of specific disorders of the blood vessels (like Klippel-Trenaunay Syndrome). Previous research indicates that changes in genes like FOXC2, thrombomodulin (THBD), and desmuslin (SYNM) could contribute to varicose veins by affecting how veins function. However, these investigations have generally involved small sample sizes, ranging from 18 to 700 participants, and there needs to be more follow-up studies to confirm these findings.
A 2019 Genome-Wide Association Study (GWAS), including nearly 10,000 cases and 300,000 controls, identified 30 genetic locations strongly linked with varicose veins. The most notable associations were found in the intron region of CASZ1 (rs1112165), previously implicated in blood pressure, and in the 16q24 region, where the PIEZO1 gene is located.
CASZ1 Gene
In the past few years, multiple GWAS have been carried out, focusing specifically on varicose veins in the lower limbs. The initial study was undertaken by 23andMe and involved European subjects who self-reported having varicose veins. This was succeeded by another GWAS that used clinically verified German cohorts. More recently, a validation study on the key findings from both studies, using independent samples from Russia and the UK Biobank, was also done.
The most compelling association was with rs11121615, a variant initially identified in the 23andMe study. This single nucleotide polymorphism (SNP) is situated within an intron of the Castor Zinc CASZ1 gene, but its functional impact remains uncertain.
PIEZO1 Gene
The association of both the CASZ1 and the PIEZO1 gene was confirmed in a significant and perhaps largest GWAS to date, conducted in 2022, focusing on surgically confirmed varicose vein cases. Another study found uncommon protein-truncating variants (PTVs) in the PIEZO1 gene that were linked to the occurrence of varicose veins. The findings indicate that while rare, these PTVs in PIEZO1 could serve as potent genetic risk factors for varicose veins.
Non-Genetic Risk Factors of Varicose Veins
While genetics can play a significant role in developing varicose veins, various non-genetic factors can also contribute. Recognizing these factors can help individuals take preventive measures. Here’s a closer look at some of the most prevalent non-genetic risk factors.
Age
As people age, their risk of developing varicose veins increases. The valves in the veins weaken over time, making it more challenging to maintain proper blood flow. While you can’t stop aging, being aware of this risk can help you take preventive steps.
Pregnancy
During pregnancy, blood volume increases, but blood flow from the legs to the pelvis decreases. This circulatory change is designed to support the growing fetus but can also result in enlarged veins in the legs.
Obesity
Carrying excess weight puts additional pressure on the veins, which can lead to varicose veins. The extra weight can also exacerbate existing conditions and make it more challenging for the veins to pump blood back to the heart.
Sedentary Lifestyle
A lack of physical activity can impair blood circulation, increasing the risk of developing varicose veins. Regular exercise helps improve blood flow and can mitigate the risk.
Occupation
Jobs that require prolonged standing or sitting can inhibit proper blood circulation and contribute to the formation of varicose veins. If your job requires long periods of immobility or air travel, taking breaks to move around is essential.
Previous Medical History
If you’ve had blood clots, injuries that affect the veins, or surgery that alters the blood flow in your legs, you may be at higher risk for varicose veins.
How To Tell If You Have Varicose Veins?
Varicose veins can manifest in various symptoms, ranging from mild to severe. Recognizing these signs and symptoms early can help you take the necessary steps to manage the condition effectively.
Visual Indicators
The most evident symptom of varicose veins is their visual appearance. The veins appear enlarged, swollen, and twisted, often with a blue or dark purple color. They are usually visible under the skin and are most commonly found in the legs and feet.
Physical Discomfort
- This can be especially pronounced after long periods of standing or sitting.
- Burning Sensation: Some individuals experience a burning or throbbing feeling around the area where the varicose veins appear.
- Muscle Cramping: Muscle cramps, particularly at night, can occur in the legs with varicose veins.
Itching: There may be an itching sensation around the vein or the surrounding skin.
Skin Changes
Over time, untreated varicose veins can lead to changes in the skin around them. This may include:
- Dry or Thinning Skin: The skin over the veins may become thin, itchy, and discolored.
- Inflammation: The skin can become red and inflamed, indicative of dermatitis, a common side effect of varicose veins.
- Ulcers: In severe cases, prolonged venous insufficiency can lead to the formation of ulcers, mainly near the ankles.
Complications
- Bleeding: The veins close to the skin may sometimes rupture and cause minor bleeding.
- Thrombophlebitis: In some cases, the varicose veins can become painful and inflamed, signaling a blood clot known as thrombophlebitis.
Aggravating Factors
Certain activities or conditions can exacerbate the symptoms, such as:
- Prolonged Standing or Sitting: Being in the same position for long durations can worsen the symptoms.
- Heat: Hot weather or warm temperatures can dilate the veins and make the symptoms more noticeable.
- Menstrual Cycle: Some women report that their symptoms worsen during their menstrual cycle due to hormonal fluctuations.
Understanding the signs and symptoms of varicose veins is crucial for early diagnosis and effective management. If you notice any of these symptoms, it is advisable to consult a healthcare provider for a comprehensive evaluation and treatment plan.
When To Worry About Varicose Veins?
Immediately consult your healthcare provider about your varicose veins if they become painful, if the skin around them changes, or if you start to develop sores or rashes near them. Another red flag is if the veins feel warm or the skin around them becomes discolored. These could be signs of a more serious issue like a blood clot or infection that needs immediate medical attention.
Further, if there is swelling in your legs or ankles, severe and unexplained pain, or if the veins start to bleed, consult a healthcare provider as soon as possible. These symptoms could indicate complications that may require prompt medical treatment.
How To Prevent Varicose Veins?
Varicose veins can cause discomfort and may even lead to more serious health complications. Prevention is always better than cure, and in the case of varicose veins, there are several steps one can take to reduce the risk or slow down their development. Below are some of the most effective prevention methods, divided into key categories for better understanding.
Lifestyle Changes
Lifestyle changes are the cornerstone of preventing varicose veins. Such changes can include:
Regular Exercises
Physical activity, especially leg exercises like walking, jogging, and swimming, can help improve blood circulation and strengthen the veins.
Consider Weight Management
Extra pressure on veins, particularly in the legs, can increase the risk of developing varicose veins. A balanced weight can help reduce this strain, although it’s important to note that weight is just one of many factors that could contribute to varicose veins. Consult your healthcare provider for a personalized plan.
Posture and Positioning
Positioning your body throughout the day can significantly affect your vein health.
Avoid Prolonged Standing or Sitting
Standing or sitting for long periods can cause blood to pool in the legs, increasing blood pressure on the veins. Make it a habit to move around every 30 minutes.
Elevate Your Legs
Elevating your legs above heart level encourages blood flow back towards the heart when resting.
Footwear Choices
The shoes you wear can have a surprisingly significant impact on your vein health.
Choose Low-Heeled Shoes
Low-heeled shoes work your calf muscles more, which is better for your veins. Avoid high heels whenever possible.
Use Compression Stockings
For those at high risk of developing varicose veins, such as people with a family history or those who stand for long periods, compression stockings can help. These stockings apply pressure to the legs and aid in blood circulation.
Dietary Changes
Your diet plays a critical role in the health of your veins.
High-Fiber and Low-Salt Diet
A diet rich in fiber can help prevent constipation, which can contribute to varicose veins. Meanwhile, a low-salt diet helps to reduce water retention, thus decreasing the pressure on your veins.
Stay Hydrated
Proper hydration can improve blood circulation, reducing the risk of blood clots and varicose veins.
Regular Health Checks
Consult Your Doctor
If you’re at higher risk for developing varicose veins due to your family history or lifestyle, it’s important to consult a healthcare provider for a personalized prevention plan. Regular check-ups can help in early detection and management.
About The LifeDNA Skincare Report
LifeDNA Skincare Report offers personalized advice on skin care routines, product recommendations, and lifestyle changes. For instance, if you are genetically prone to tanning quickly, the report might suggest a higher SPF sunscreen and additional protective measures like wearing hats and long sleeves.
The LifeDNA Skincare report covers an analysis of Varicose Veins. Get yours here.
Summary
- Varicose veins are a common but often preventable circulatory issue affecting many adults.
- Genetic and lifestyle factors influence the risk, but early intervention and proper management can alleviate the symptoms and complications.
- Early symptoms like pain, discoloration, and inflammation can help effectively treat and prevent complications.
- Several GWAS on varicose veins have established prospective genetic markers influencing varicose veins.
- Non-genetic risk factors like sedentary lifestyle, type of occupation, and footwear choices can also influence predisposition to varicose veins.
*Understanding your genetics can offer valuable insights into your well-being, but it is not deterministic. Your traits can be influenced by the complex interplay involving nature, lifestyle, family history, and others.
Our reports and suggestions do not diagnose or treat any health conditions or provide any medical advice. Consult with a healthcare professional before making any major lifestyle changes or if you have any other concerns about your results.
References
- https://www.nhsinform.scot/illnesses-and-conditions/heart-and-blood-vessels/conditions/varicose-veins
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3217733/
- https://www.nhlbi.nih.gov/health/varicose-veins
- https://www.cuimc.columbia.edu/news/when-worry-about-varicose-veins
- https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.118.035584?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6400474/
- https://pubmed.ncbi.nlm.nih.gov/8176043/
- https://blog.23andme.com/wp-content/uploads/2014/10/Bell_ASHG2014_varicose.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5379489/
- https://pubmed.ncbi.nlm.nih.gov/29660117
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6769056/
*Understanding your genetics can offer valuable insights into your well-being, but it is not deterministic. Your traits can be influenced by the complex interplay involving nature, lifestyle, family history, and others.
Our reports have not been evaluated by the Food and Drug Administration. The contents on our website and our reports are for informational purposes only, and are not intended to diagnose any medical condition, replace the advice of a healthcare professional, or provide any medical advice, diagnosis, or treatment. Consult with a healthcare professional before making any major lifestyle changes or if you have any other concerns about your results. The testimonials featured may have used more than one LifeDNA or LifeDNA vendors’ product or reports.