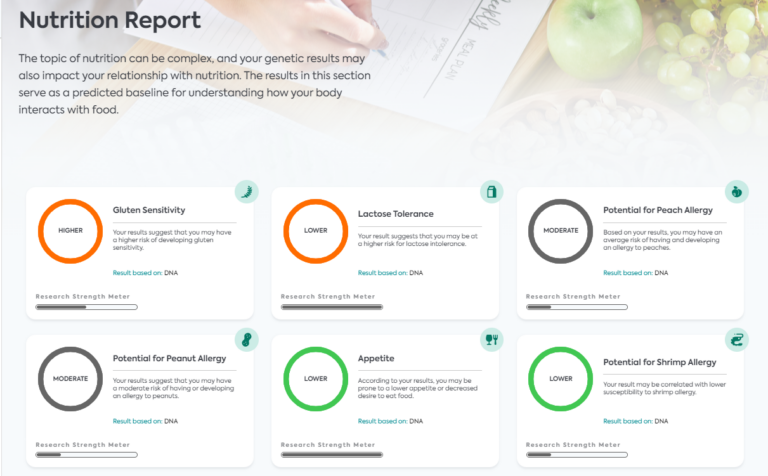
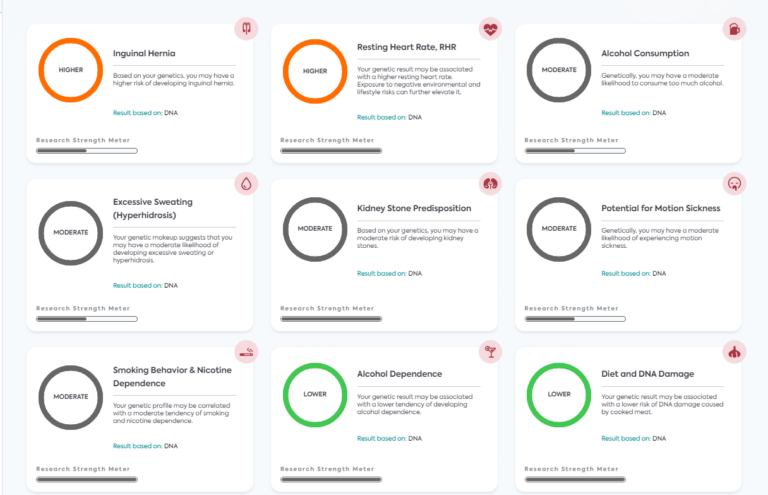
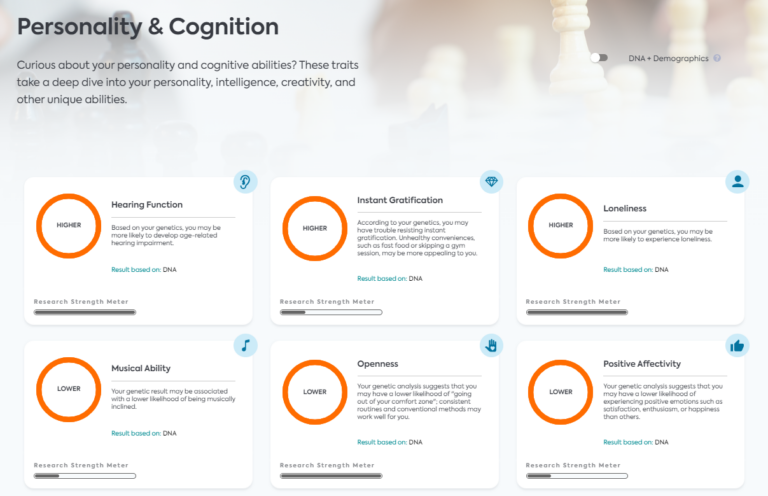
Understanding the Role of Genetics in Carbohydrate Metabolism
Aira
on
August 16, 2024
Overview
What is Carbohydrate Metabolism?
The word “carbohydrate” has been through the mud in recent years. From being an important component in human and many animal diets to becoming an enemy of sorts for fear of gaining weight when you eat them, carbs are still around and will continue to be here in the foreseeable future. That is a good thing, considering how important carbohydrates are.
By definition, the body uses carbohydrates as its main energy source. They are converted into glucose, a form of sugar when ingested, and glucose is then released into the bloodstream. Then, the cells may immediately utilize the glucose for energy or store it as glycogen in the muscles and liver for later use. Carbs are usually classified into two groups:
- Simple Carbohydrates: Simple carbohydrates, also known as “sugars,” break down quickly and can result in sharp rises in blood sugar levels. They can be a quick source of energy due to their quick digestion, but it is advisable to eat them in moderation since an excessive amount might have negative effects on your health.
- Complex Carbohydrates: The body breaks down complex carbs, often known as “starches,” more slowly, resulting in a prolonged release of energy and aiding in the stabilization of blood sugar levels. Compared to simple sugars, complex carbs are healthier since they are a good source of fiber and minerals.
Carbohydrate metabolism is the process through which the body breaks down the carbs that are absorbed. The body goes through a series of biochemical processes to break down carbs into glucose and other simpler molecules, which can then be used as fuel. It is a fundamental biological procedure that is crucial in giving living things energy. The proper operation of cells, tissues, and organs depends on this energy.

How Does Carbohydrate Metabolism Work?
The process of carbohydrate metabolism can be broadly divided into two main phases:
- Glycolysis: The cytoplasm of cells is where this first stage of glucose metabolism takes place. One molecule of glucose is split into two molecules of pyruvate during the process of glycolysis. This process produces a small amount of adenosine triphosphate (ATP), which is a kind of energy that doesn’t require oxygen.
- Cellular Respiration: Pyruvate enters the mitochondria if oxygen is present, where it engages in a series of intricate events to generate a sizable amount of ATP. The Krebs cycle and the electron transport chain are both involved in this process. For the majority of cellular processes, the ATP produced serves as the main energy source.
The metabolism of carbohydrates is not just for energy production. For later energy needs, extra glucose is stored as glycogen in the muscles and liver. Glycogen can be turned back into glucose through a process known as glycogenolysis when blood glucose levels fall.
Carbohydrate metabolism that is out of balance can have detrimental effects on health, including diabetes, where the body’s capacity to control blood sugar is compromised. For sustaining general health and energy balance, it is essential to understand and control glucose metabolism. It also has a significant impact on different facets of exercise, diet, and metabolic problems.
What Influences Carbohydrate Metabolism?
Carbohydrate metabolism is a complex process influenced by various factors. These factors can impact how the body processes, utilizes, and regulates carbohydrates.
Genetic Factors Influencing Carbohydrate Metabolism
The metabolism of carbohydrates can be strongly influenced by genetic variables. An individual’s risk of developing diseases like diabetes, and their reaction to various forms of carbs may vary depending on genetic variations in the genes involved in producing insulin, absorbing glucose, and other elements of carbohydrate metabolism.
Insulin Regulation
Variations in genes related to insulin production and function can affect an individual’s ability to regulate blood sugar levels. For example, the TCF7L2 gene (SNP rs12255372) is associated with an increased risk of type 2 diabetes, as it can influence insulin secretion and sensitivity.
Glucose Transporters
Genes coding for glucose transporters, such as the GLUT2 and GLUT4 genes, play a role in how the body moves glucose into cells. Variations in these genes can impact the efficiency of glucose uptake.
Glycogen Storage
Genes related to the storage and utilization of glycogen, the storage form of glucose in the liver and muscles, can influence an individual’s glycogen levels and response to carbohydrate intake.
Metabolic Enzymes
Genetic variations in enzymes that are involved in carbohydrate metabolism, such as amylase (important for breaking down starches), can affect how efficiently carbohydrates are digested.
Lipid Metabolism
Some genes involved in lipid (fat) metabolism, such as those related to triglycerides and cholesterol, can indirectly influence carbohydrate metabolism, as lipid metabolism and carbohydrate metabolism are interconnected.
Other Genetic Markers
A genetic marker for carbohydrate metabolism that is included in LifeDNA’s report is SNP rs17046216 (SC4MOL) which is under-expressed in an animal model of T2D and plays a key role in lipid biosynthesis, with implications for the regulation of energy metabolism, obesity, and dyslipidemia. Another SNP rs10829848 (TCERG1L) is associated with plasma adiponectin, a key modulator of obesity, inflammation, IR, and diabetes. More SNPs included are rs7607980 (COBLL1/GRB14), rs2943634 (IRS1), rs4691380 (PDGFC), rs4841132 (PPP1R3B), rs77465890 (CSMD1), rs780094 (GCKR), and rs35767 (IGF1).
While genetics play a part in how our bodies process carbohydrates, environmental factors such as diet and exercise also play a role. Lifestyle decisions are extremely important in controlling glucose metabolism and general health because they frequently minimize the effects of inherited predispositions.
Understanding one’s genetic profile can offer insightful information about how an individual may react to various dietary approaches and assist in customizing recommendations for optimal carbohydrate metabolism, especially in the context of personalized nutrition and health.
Non-Genetic Factors Influencing Carbohydrate Metabolism
Non-genetic factors play a significant role in influencing carbohydrate metabolism. These factors can impact how the body processes, utilizes, and regulates carbohydrates.
Diet and Nutrition
- Type of Carbohydrates: The types of carbohydrates consumed, including simple sugars, complex carbohydrates, and dietary fiber, can affect how carbohydrates are metabolized. Simple sugars can lead to rapid spikes in blood sugar, while complex carbohydrates and fiber offer more sustained energy.
- Dietary Fiber: A high-fiber diet can slow the digestion and absorption of carbohydrates, promoting better blood sugar control and satiety.
- Processed Foods: Diets high in processed and refined carbohydrates, such as sugary snacks and beverages, can contribute to rapid fluctuations in blood sugar levels.
Physical Activity
Exercise: Regular physical activity improves insulin sensitivity, making it easier for the body to regulate blood sugar levels. Exercise also increases the muscles’ ability to use glucose for energy.
Hormones and Health Conditions
- Insulin Resistance: Insulin resistance, a condition where cells do not respond effectively to insulin, can disrupt carbohydrate metabolism. It is often associated with obesity and sedentary lifestyles.
- Hormonal Changes: Conditions like polycystic ovary syndrome (PCOS) and hormonal changes during pregnancy can affect carbohydrate metabolism.
Stress
Chronic Stress: Chronic stress can elevate cortisol levels, which, in turn, can impact blood sugar regulation. Stress management is important for maintaining healthy carbohydrate metabolism.
Medications
Prescription and OTC Drugs: Certain medications, such as corticosteroids and antipsychotic drugs, can affect carbohydrate metabolism and increase the risk of insulin resistance.
Age
Aging: This very natural process of life is associated with changes in carbohydrate metabolism. Older individuals may experience reduced insulin sensitivity.
Metabolic Rate
Metabolism: An individual’s metabolic rate, influenced by factors like genetics and activity level, can affect the rate at which carbohydrates are burned for energy.
For a balanced glucose metabolism and overall well-being, it is crucial to comprehend and control these non-genetic elements. The right lifestyle choices can be extremely important for optimizing glucose metabolism. These include a balanced diet, frequent exercise, and stress management.
How is Carbohydrate Metabolism Related to Weight Management?
A person may have a tougher problem digesting carbohydrates and converting them into the energy they need to go through the day depending on their metabolic rate. Carbohydrate metabolism testing can also reveal important information about weight control, such as the need to limit processed sweets and increase the intake of complex carbohydrates to fuel the body.
While simple sugars may provide immediate energy, they are more likely to result in fluctuations in weight management because they are less satisfying, have little nutritional value, affect hormones and blood sugar levels, and make people eat more than they intended to.
This is not to imply that one cannot still indulge in their favorite chocolate, but moderation is always the key to a healthy, balanced diet. Think about switching to complex carbohydrates like quinoa, sweet potatoes, oatmeal, and brown rice. If taken in moderation, they have greater nutritional content and can offer a constant supply of energy throughout the day.
What is Carbohydrate Metabolism Disorder?
The set of rare genetic illnesses in carbohydrate metabolism often referred to as carbohydrate metabolic disorders or inborn errors of metabolism, affects how well the body processes and uses carbohydrates.
Mutations in particular genes that encode enzymes or proteins involved in various areas of glucose metabolism cause these illnesses. As a result, people with these illnesses may struggle to adequately digest, absorb, or use carbohydrates. Some of the most common metabolic disorders include:
- Galactosemia: Galactose, a sugar included in milk and dairy products, cannot be adequately metabolized by the body as a result of this condition. If not treated with a strict galactose-free diet, it can result in serious health issues.
- Fructose Intolerance: The natural sugar fructose, which can be found in honey and fruits, cannot be effectively processed by people who have fructose intolerance. Foods high in fructose can cause symptoms such as liver damage and digestive troubles.
- Glycogen Storage Diseases: These illnesses impair the body’s capacity to hold onto and release glycogen, a kind of glucose that is stored in the body. A glycogen storage disease may cause issues with low blood sugar (hypoglycemia), muscle weakness, and other symptoms, depending on the precise variety.
Genetic testing and clinical assessments are frequently used to diagnose abnormalities in carbohydrate metabolism. Dietary management is often used as part of treatment to limit or adjust carbohydrate intake while advising to avoid foods or sweets that the patient cannot effectively digest.
Healthcare personnel should continuously monitor the disorder management and treatment to avoid complications and guarantee the patient’s well-being. Even though these conditions are uncommon, if neglected, they can have detrimental effects on one’s health. For people with abnormalities of carbohydrate metabolism, early diagnosis and appropriate care are crucial.
Symptoms
Carbohydrate metabolic disorders, also known as inborn errors of metabolism, can manifest with a wide range of symptoms, which can vary depending on the specific disorder, its severity, and the age of onset. Some common symptoms and signs of carbohydrate metabolic disorders may include:
- Hypoglycemia (Low Blood Sugar): Shakiness, sweating, weakness, paleness, irritability, rapid heartbeat, lethargy, and seizures.
- Vomiting and Gastrointestinal Issues: Nausea, abdominal pain, diarrhea, poor feeding (in infants), and failure to thrive (poor weight gain and growth).
- Liver Problems: Enlarged liver (hepatomegaly), jaundice (yellowing of the skin and eyes), and elevated liver enzymes.
- Kidney Problems: Kidney dysfunction and high levels of substances in the blood that should be excreted by the kidneys.
- Hemolytic Anemia: Pale skin, fatigue, dark urine (due to the breakdown of red blood cells), and enlarged spleen (splenomegaly).
- Neurological Symptoms: Seizures, developmental delays, intellectual disabilities, abnormal muscle tone, movement disorders, and behavioral issues.
- Metabolic Acidosis: Increased acidity in the blood, leading to symptoms like rapid breathing, confusion, and lethargy.
- Lactic Acidosis: High levels of lactic acid in the blood, which can cause muscle pain, rapid breathing, and fatigue.
Depending on the underlying genetic abnormality and the person’s age, the specific symptoms can change. Some metabolic abnormalities related to carbohydrates are present in infants, whereas others may show up later in childhood or even as adults. The management and treatment of these conditions require an early diagnosis in order to reduce complications and enhance the quality of life for the patient.
Naturally Boosting Your Carbohydrate Metabolism
Improving and optimizing carbohydrate metabolism is essential for everybody’s overall health, energy levels, and weight management. While genetic and non-genetic factors can influence your carbohydrate metabolism, there are several natural strategies to help boost it.
Balanced Diet
- Consume a balanced diet that includes a variety of complex carbohydrates, such as whole grains, fruits, vegetables, legumes, and nuts.
- Limit the intake of refined sugars and processed carbohydrates, as they can lead to rapid spikes in blood sugar.
- Stay well-hydrated, as dehydration can affect blood viscosity and circulation, potentially impacting carbohydrate metabolism.
- Be mindful of portion sizes. Eating in moderation helps maintain stable blood sugar levels and prevents overconsumption of carbohydrates.
- Include protein in your meals and snacks. Protein can help regulate blood sugar and increase feelings of fullness, reducing the risk of overeating carbohydrates.
Fiber-Rich Foods
- Prioritize foods high in dietary fiber. Fiber slows down the digestion and absorption of carbohydrates, leading to better blood sugar control and increased feelings of fullness.
- Whole grains, oats, beans, and fruits and vegetables are excellent sources of fiber.
Regular Physical Activity
- Engage in regular physical exercise, including cardiovascular activities (e.g., walking, jogging, cycling) and strength training.
- Exercise improves insulin sensitivity, making it easier for the body to utilize carbohydrates effectively.
- Consider incorporating high-intensity interval training (HIIT) into your exercise routine. HIIT has been shown to improve glucose metabolism and insulin sensitivity.
Adequate Sleep and Rest
- Prioritize good-quality sleep. Sleep deprivation can lead to insulin resistance and affect blood sugar control.
- Manage stress through techniques such as mindfulness, meditation, yoga, or deep breathing exercises. Chronic stress can impact blood sugar regulation.
Regular Health Check-ups
- Get regular health check-ups to monitor your blood sugar levels and overall metabolic health. Early detection of any metabolic issues is crucial for prompt intervention.
- If you have concerns about your carbohydrate metabolism or are at risk for metabolic disorders, consult a healthcare professional, such as a registered dietitian or endocrinologist, for personalized guidance and recommendations.
Individual reactions to various techniques for improving carbohydrate metabolism may differ. Consult a medical expert or qualified dietitian for individualized advice and suggestions if you have questions regarding your carbohydrate metabolism. They can assist in modifying a strategy to fit your unique requirements and objectives.
Personalized Nutrition: LifeDNA’s Carbohydrate Metabolism Report
Your body may have a quick metabolism, which increases the likelihood that your metabolism of carbohydrates is likewise healthy. It is possible to increase your metabolism with a healthy diet and regular exercise, but it is vital to remember that these are not your only options. How your body handles carbs can also be determined by your genetic variants.
LifeDNA’s Carbohydrate Metabolism Report can tell you how well your body responds to carbohydrate intake. This can help you with your nutrition goal, whether that is weight management, lowering your blood sugar levels, or simply maintaining a healthy body. Try LifeDNA today.
Summary
- Carbohydrates are one of the body’s primary energy sources. When consumed, carbohydrates turn into glucose, a type of sugar, which is subsequently released into the bloodstream.
- The body’s biological reactions break down ingested carbohydrates into glucose and other simpler molecules, which can subsequently be used as fuel for cells. This process is known as carbohydrate metabolism.
- Unbalanced carbohydrate metabolism can have negative implications on health, including diabetes.
- The intricate process of carbohydrate metabolism is controlled by a number of variables. These elements may affect how the body breaks down, uses, and controls carbs.
- Different methods for boosting carbohydrate metabolism may have different effects on different people. If you have any questions about your carbohydrate metabolism, speak with a medical professional or a competent nutritionist for personalized guidance and recommendations.
References
- https://medlineplus.gov/carbohydrates.html#:~:text=What%20are%20carbohydrates%3F,cells%2C%20tissues%2C%20and%20organs.
- https://www.houstonmethodist.org/blog/articles/2023/jan/simple-vs-complex-carbs-are-simple-carbs-always-bad-are-complex-carbs-always-healthier/#:~:text=What%20are%20simple%20carbohydrates%3F,added%20to%20many%20processed%20foods.
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/carbohydrate-metabolism
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7778149/
- https://accessmedicine.mhmedical.com/content.aspx?bookid=2355§ionid=185844537#:~:text=Glycolysis%20is%20the%20metabolic%20pathway,(Figure%206%2D2).
- https://pubmed.ncbi.nlm.nih.gov/23680095/#:~:text=This%20process%20is%20called%20cellular,in%20the%20form%20of%20monosaccharides.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3662485/#:~:text=Carbohydrate%20metabolism%20in%20humans%20is,and%20suppresses%20hepatic%20glucose%20production.
- https://nutritionj.biomedcentral.com/articles/10.1186/s12937-022-00813-w
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7462924/
- https://www.ncbi.nlm.nih.gov/books/NBK459277/
- https://www.sciencedirect.com/science/article/abs/pii/S1751722222001895#:~:text=Carbohydrates%20are%20typically%20broken%20down,normoglycaemia%20in%20times%20of%20fasting.
- https://courses.lumenlearning.com/suny-ap2/chapter/carbohydrate-metabolism-no-content/#:~:text=Metabolic%20enzymes%20catalyze%20catabolic%20reactions,or%20glycogen%20for%20later%20use.
- https://pubmed.ncbi.nlm.nih.gov/2436011/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5629314/#:~:text=Lipid%20and%20carbohydrate%20metabolism%20are,sugars%2C%20and%20amino%20acid%20precursors.
- https://medlineplus.gov/carbohydratemetabolismdisorders.html
- https://effectivehealthcare.ahrq.gov/health-topics/carbohydrate-metabolism-disorders#:~:text=Carbohydrate%20metabolism%20disorders%20are%20a,enzymes%20may%20not%20work%20properly.
- https://rarediseases.org/rare-diseases/galactosemia/
- https://www.webmd.com/digestive-disorders/what-is-fructose-intolerance
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/glycogen-storage-disease#:~:text=disease%20in%20children-,Glycogen%20storage%20disease%20(GSD)%20is%20a%20rare%20condition%20that%20changes,show%20any%20signs%20of%20GSD.
- https://onlinelibrary.wiley.com/doi/10.1111/ijpo.12765
- https://www.nature.com/articles/ng.2274
- https://academic.oup.com/hmg/article/21/20/4530/655461
- https://www.nature.com/articles/ng.520
*Understanding your genetics can offer valuable insights into your well-being, but it is not deterministic. Your traits can be influenced by the complex interplay involving nature, lifestyle, family history, and others.
Our reports have not been evaluated by the Food and Drug Administration. The contents on our website and our reports are for informational purposes only, and are not intended to diagnose any medical condition, replace the advice of a healthcare professional, or provide any medical advice, diagnosis, or treatment. Consult with a healthcare professional before making any major lifestyle changes or if you have any other concerns about your results. The testimonials featured may have used more than one LifeDNA or LifeDNA vendors’ product or reports.