Are Keloid Scars Genetic?
Catherine
on
March 5, 2025
Disclaimer: This article is for informational purposes only and is not intended for diagnostic use. LifeDNA does not provide diagnostic reports on any traits discussed. Genetics is just one piece of the puzzle; please consult a healthcare professional for comprehensive guidance on any health condition.
Why do some scars grow uncontrollably, becoming thick, raised, and itchy long after a wound has healed? For some people, even a small cut, acne breakout, or piercing may lead to a type of scar called a keloid. Unlike typical scars that fade over time, keloids can expand far beyond the original injury, becoming larger, firmer, and sometimes very uncomfortable.
Since keloids can be painful, itchy, or cause emotional distress, especially when they appear in visible areas, knowing what triggers them helps guide better management and care.
What Is a Keloid Scar?
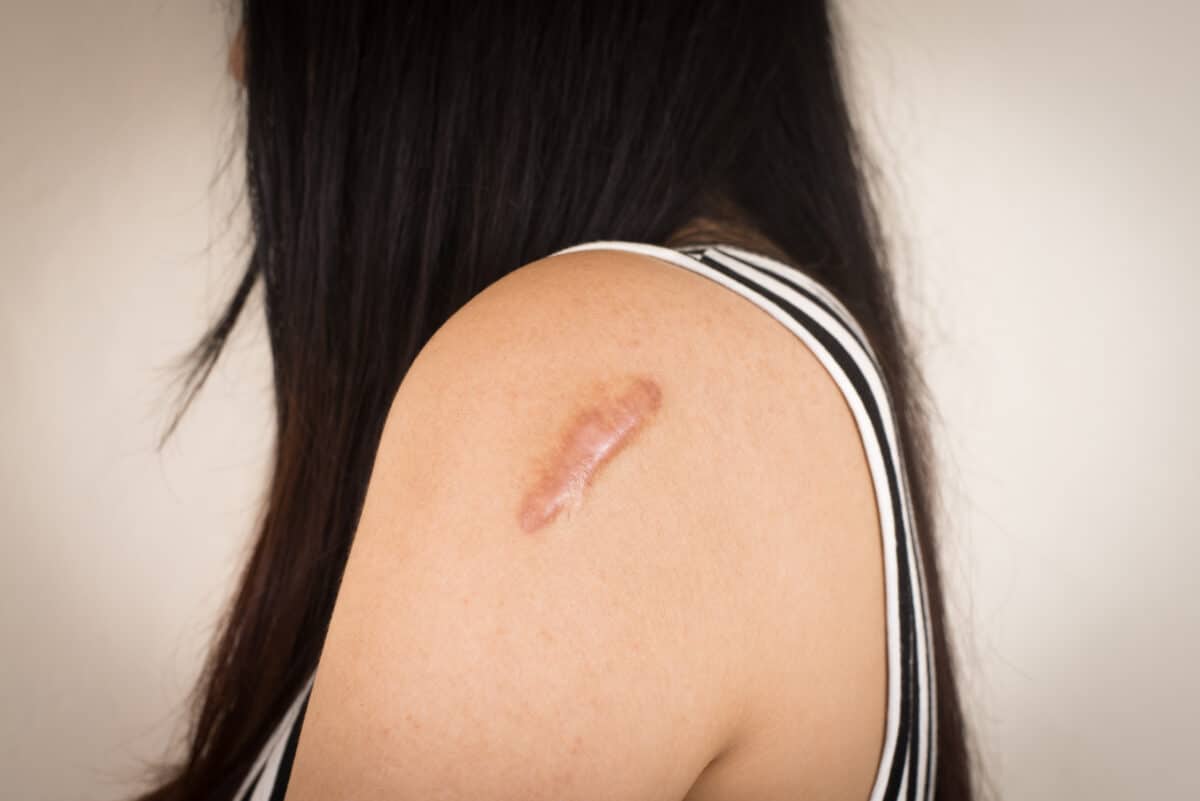
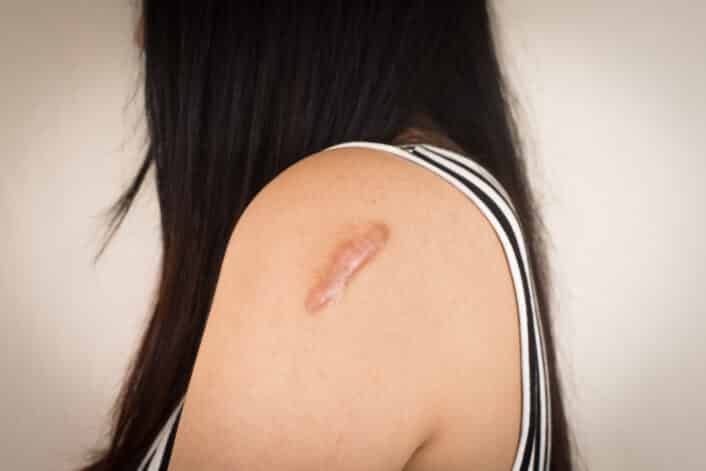
A keloid is a type of raised scar that grows too much after a skin injury. Unlike regular scars that stop growing once the skin heals, keloids keep expanding beyond the edges of the original wound. They may appear thick, rubbery, and sometimes red or darker than the surrounding skin. Keloids are not dangerous. But they may cause discomfort, itching, or emotional stress because of how they look.
These scars commonly appear on the earlobes, shoulders, cheeks, or chest, but they can develop on any part of the body. People who are more prone to keloids may notice them forming in more than one location.
What Causes Keloid Scars?
Keloids are caused by a combination of genetic and environmental factors. The genetic predisposition to form keloids means that individuals with a family history of keloids are more likely to develop them, especially when exposed to certain environmental triggers like skin injuries.
Common triggers include skin injuries such as acne outbreaks, body piercings, burns, surgical wounds, and even minor cuts or scratches. Other environmental factors like infections, skin irritation, and hormonal changes which may occur during puberty, pregnancy, or with certain medical conditions may increase the risk of keloid formation.
What Are the Symptoms of a Keloid Scar?
Keloids are most common on areas like the chest, shoulders, back, and earlobes, but can form anywhere on the body. Keloid scars may develop months or even years after an injury. Here are the common symptoms:
- Thick, irregular growth: Keloid scars grow beyond the boundaries of the original wound, creating a thick and uneven surface.
- Shiny, hairless surface: The skin over the keloid is smooth and lacks hair.
- Firm or rubbery texture: Keloids can feel tough or rubbery when touched, and may be more raised compared to the surrounding skin.
- Variable size: The size of a keloid depends on the size of the initial injury and how long the scar continues to grow.
- Color: Keloids may appear reddish, purplish, or brown, often darker than the surrounding skin, depending on skin type.
- Itching or discomfort: Keloids may cause itching or a sensation of discomfort, especially during growth.
- Tightness near joints: If a keloid forms near a joint, it may cause tightness, restricting movement.
Genetics and Keloid Scars
Beyond family history, several genetic studies have connected keloids with specific genes and inherited conditions.. While no single factor has been found to cause keloids on its own, multiple genetic factors play a role in increasing the risk. Studies show that several genes involved in collagen production, immune response, and wound healing are linked to keloid development.
A key factor in keloids is the overproduction of collagen, a protein that helps give the skin its structure. Research has shown that the cells responsible for making collagen in keloids, called fibroblasts, are more active than those in normal skin. This leads to too much collagen being produced, which causes the raised, thick scars that are characteristic of keloids. Studies on gene expression in keloid tissues found that levels of TGF-β1 and TGF-β2 are higher than normal, leading to excessive collagen production. TGF-β is a growth factor that helps skin heal, and its overactivity is a major factor in keloid formation..
Further research has shown that so-called SMAD genes, which are involved in the TGF-β signaling process, also play a role in keloid formation and specifically SMAD2 and SMAD3 genes are involved in the abnormal collagen production seen in keloids. When these genes’ regulation is disrupted, it leads to excessive collagen being produced, contributing to the formation of keloids.
Genome-wide studies have found certain novel regionsof the genome that might be linked to keloid development. GWAS studies on Japanese and African-American families have found regions on chromosomes 1, 3, and 15 that could increase the risk of keloids. Replication studies identified the NEDD4 gene on chromosome 15 involved in collagen production, suggesting it may contribute to keloid formation.
In addition to these genes, variants in HLA genes, like HLA-DRB1*15, have been linked to keloids. Studies on HLA-DRB1 genes’ association have shown that this gene may affect how the immune system responds during wound healing, possibly leading to abnormal scar tissue. This was demonstrated in studies involving the HLA-DRB1*15, which were replicated in both Chinese and Caucasian ethnic groups, suggesting this haplotype increases the risk of keloids in these populations. Epigenetic studies on keloid fibroblasts have found that these cells have altered DNA methylation and histone acetylation patterns. These changes could influence gene activity, contributing to the abnormal scarring process seen in keloids.
Is Keloid Scars Inheritable?
Keloid scars can be inherited. They often run in families and are more common in people with African, Asian, or Hispanic backgrounds. In some cases, inheriting just one copy of a gene variant (autosomal dominant inheritance) may increase risk, while for other genes both variant copies may be needed (autosomal recessive inheritance).
Is Keloid Scars Treatable?
Keloid scars can be treated, though they may return even after treatment. The approach often depends on the size, location, and how long the scar has been present. Treatment options include:
- Corticosteroid injections, which help reduce inflammation and flatten the keloid over time
- Silicone gel sheets or pads, which soften the scar and may help reduce its size
- Laser therapy, which can decrease both the thickness and discoloration of the scar
- Cryotherapy, where the keloid is frozen using liquid nitrogen; most effective for smaller scars
- Surgical removal, which physically cuts out the scar, usually followed by other treatments to prevent it from coming back
- Radiation therapy, sometimes used after surgical removal to lower the chance of regrowth
Can Keloid Scars Be Prevented?
It is not always possible to prevent keloid scars, especially if you’re genetically more likely to develop them. Here are some ways to protect your skin and reduce the chance of keloid scars:
- Avoid unnecessary skin trauma: Try to skip piercings, tattoos, or other procedures that break the skin if you’re prone to keloids.
- Treat skin issues early: If you have acne or skin infections, manage them quickly to prevent them from turning into deeper wounds that may scar.
- Use protective coverings after piercings: If you do get your ears pierced, wearing pressure earrings or silicone pads can help flatten the area and reduce the chance of a keloid forming.
- Keep wounds clean and protected. Clean your wounds gently and keep them moist with ointment so they can heal more smoothly.