
Disclaimer: This article is for informational purposes only and is not intended to diagnose any conditions. LifeDNA does not provide diagnostic services for any conditions mentioned in this or any other article.
Sickle cell anemia is a well-known inherited blood disorder. Around 300,000 babies are born with sickle cell disease each year worldwide. The majority of people with sickle cell disease live in sub-Saharan Africa, India, the Mediterranean region, and the Middle East. In the United States, about 100,000 individuals are affected by the condition. It is caused by a genetic mutation that changes the shape and behavior of red blood cells (RBCs), leading to a range of health problems. Understanding the genetics behind sickle cell anemia helps explain how the disease is passed down, why it varies in severity, and how genetic screening can be used to manage it.
Sickle cell anemia is caused by a mutation in the HBB gene, which provides instructions for making a part of hemoglobin — the protein in red blood cells that carries oxygen. This mutation changes just one DNA base, replacing adenine (A) with thymine (T). As a result, a single amino acid in the hemoglobin protein changes from glutamic acid to valine at position 6 of the beta-globin chain. This altered form of hemoglobin is called hemoglobin S (HbS).
Also read: The Genetics of beta-Thalassemia
Hemoglobin is a vital protein found in RBCs, and its main job is to carry oxygen from the lungs to the rest of the body. Every cell needs oxygen to produce energy, and hemoglobin ensures that oxygen is delivered efficiently. Once it drops off oxygen, hemoglobin also helps carry carbon dioxide, a waste product, back to the lungs to be exhaled.
Without enough healthy hemoglobin, the body’s tissues and organs don’t get the oxygen they need, leading to fatigue, weakness, and more serious problems. In short, hemoglobin keeps your cells alive and your body functioning properly.
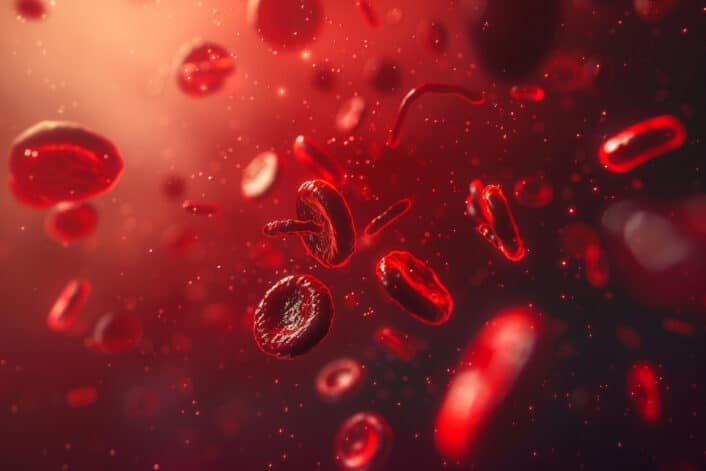
The shape of RBCs is important because it helps them do their job well. Normal RBCs are round and flexible with a flattened, disc-like shape. This shape gives them a large surface area to carry more oxygen and allows them to squeeze through tiny blood vessels without getting stuck or damaged.
In people with sickle cell anemia, hemoglobin S (HbS) sticks together when oxygen levels are low. This causes red blood cells to become stiff and take on a crescent or “sickle” shape. These misshapen cells can block blood flow, break apart easily (leading to anemia), and cause pain, organ damage, or stroke.
Sickle cell anemia follows an autosomal recessive inheritance pattern. This means a person must inherit two copies of the HbS gene mutation — one from each parent — to have the disease.
For two parents who both have sickle cell trait (one mutated copy), each child has:
Not all people with sickle cell disease have the same symptoms. Some differences are due to other genetic factors. For example:
The BCL11A gene plays a key role in sickle cell disease by controlling the type of hemoglobin the body produces. Normally, babies are born with HbF, which is later replaced by adult hemoglobin (HbA). In people with sickle cell disease, the adult hemoglobin includes a faulty version called HbS.
BCL11A acts as a repressor—it switches off the production of HbF after birth. Scientists have discovered that reducing or turning off BCL11A can reactivate HbF production, even in adults. Increasing HbF levels helps because fetal hemoglobin prevents sickling of red blood cells, reduces symptoms, and improves overall blood health.
For this reason, BCL11A is a major target in gene therapy approaches for treating sickle cell disease. By silencing or knocking down BCL11A, therapies can boost HbF production and help protect patients from the damaging effects of HbS.
In a 2020 pilot study six patients with sickle cell disease were monitored for at least six months after receiving BCH-BB694 gene therapy, which uses shmiR-based knockdown to reduce BCL11A expression. The therapy led to strong and stable increases in fetal hemoglobin (HbF), with 20.4% to 41.3% of total hemoglobin being HbF and 58.9% to 93.6% of red cells showing HbF expression. During follow-up, sickle cell symptoms were reduced or disappeared. The results support BCL11A inhibition as a promising strategy for increasing HbF and show that this gene therapy approach may offer a good balance between safety and effectiveness.
Sickle cell anemia is usually diagnosed with a blood test, often done in newborn screening programs. The test checks for different types of hemoglobin, including HbS. If a child is found to have the disease or trait, follow-up genetic testing can confirm the diagnosis and help understand the type of sickle cell condition.
Carrier testing for sickle cell trait is also important for couples planning a family, especially if both partners are from high-risk populations. Genetic counseling can help them understand the risks and options.
The sickle cell gene mutation remains common in some regions because of a natural advantage it provides. People with sickle cell trait are more resistant to severe malaria, especially from Plasmodium falciparum. This survival benefit explains why the mutation has persisted in areas where malaria is or was widespread.
Sickle cell anemia is a classic example of how a single gene mutation can lead to a serious health condition when inherited in a specific way. Advances in genetics have deepened our understanding of the disease and opened doors to new treatments, such as gene therapy and precision medicine. With early diagnosis, ongoing care, and better genetic awareness, people with sickle cell disease can live longer, healthier lives.
Read about other genetic conditions:


